Research
The molecular, cellular, and circuit mechanisms underlying autism, neuropsychiatric disorders, and epilepsy
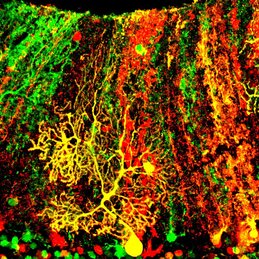
We are broadly interested in neuropsychiatric disorders with a focus on Smith-Magenis syndrome (SMS), a childhood brain disorder associated with intellectual disability, epilepsy, obesity, autism, and neuropsychiatric features. SMS is caused by 17p11.2 deletion or loss of the Retinoic Acid Induced 1 (RAI1) gene, providing an excellent genetic entry point to understand how copy number variations (CNVs) control brain development and function. The Huang lab uses genetic, molecular, cell biological, and modern stem cell and neuroscience techniques to (1) determine the molecular and neurobiological functions of RAI1/17p11.2 deletions, and (2) develop disease-modifying therapies to reverse SMS.
We are currently interested in the following questions:
We are currently interested in the following questions:
- How does RAI1 dosage imbalance in different neuronal subtypes drives molecular and behavioral deficits?
- How does RAI1 haploinsufficiency affect neuronal development?
- What are the molecular and cellular function of RAI1-containing chromatin complex?
- What are the RAI1 upstream and downstream molecules that can serve as therapeutic targets for SMS?
For our published work, please visit the PUBLICATIONS page